Determining Al release from dental cartridges and glass tubing. An effective and convenient tool to discriminate among diverse glass tubing
manufacturing processes
As described in the previous article titled, “How tubular glass manufacturing process can affect the final product stability and technological improvement to mitigate it. Al – Epinephrine interaction: a related example,” the Aluminum release from inner surfaces of glass containers becomes a key factor when the shelf life (or activity) of the contained API results to be highly sensitive to Aluminum concentration, and in many cases, the release of this element could be dramatically correlated to the glass tubing manufacturing process. To be able to discriminate which glass manufacturing process might guarantee best performance, it is necessary to define a strong enough analytical protocol (routine) starting from sample selection, sample preparation and correct manipulation (to avoid any unwanted contamination), passing through the definition of correct analytical set points (reagents, temperatures & times, instrument choice) and finally, the definition of an evaluation grid with limits and an acceptability range.
In general, to obtain an effective analytical method optimization, it is necessary to define a correct Design Of Experiment (DOE) considering all the possible direct variables that could affect the results or performance and then, determine levels of variables to be investigated (reasonably based on experience and knowledge of process variability); the result will be a Design Matrix for the factors being investigated which gives the number of trials needed.
The described methodology, voluntarily reported as a general overview and not as a detailed procedure, has been developed after testing different sets of glass tubing, produced with 3 different manufacturing processes (Vello, Coated Danner, Uncoated Danner) and the respective sets of cartridges, performing analysis with different analytical equipment and different extractable solutions (more than 180 different samples analysed). Conditions set up in order to simulate the aging and consequently to promote the release in acceptable time, involved the sterilization cycle, performed by using an autoclave in the following conditions: 60 min, 121 +/- 1 °C, 1 bar, commonly used to test the Hydrolytic Resistance of Glass Containers (Eu.Ph. 3.2.1).
Critical factors being faced are:
- The addition of concentrated acid to the extract solution in order to stabilize the signal over time.
- Pre-handling of reusable tools and equipment (silicone stoppers and auxiliary glassware must be preventively autoclaved at least 3 times) to avoid undesired contamination.
Reagents and tools (minimum requirements)
- Autoclave with temperature control and certified logger, able to perform Eu. Ph. Sterilization cycle as following described: 60 min; 121+/- 1 °C; 1 bar
- Water R1 as per Eu.Ph. definition.
- AAS with Al dedicated lamp (ICP techniques also investigated)
- Water solution of EDTA (500 ppm chosen)
- Stoppers for Glass dental cartridges Concentrate Acid (Acetic preferred)
Tools pre-handling
Tools and glass equipment that will be in contact with the extraction solution have to be washed and rinsed with Water R1 and consequently autoclaved 3 times, under Eu.Ph. conditions.
Sample preparation and manipulation
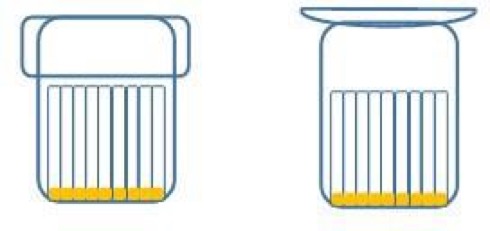
- Glass Tubings: from each glass tubing obtain 2 samples, of approx. 440 mm length, bottoming on end by using a medium flame to avoid high transforming stress in the glass. Wash samples as per Eu.Ph. and fill with EDTA Solution up to 20 mm under the neck. Group sticks with rubber rings and cover with glass beakers, identifying different samples.

- Glass cartridges: select randomly from an adequate number of cartridges, close the bottom with appropriate 3-times autoclaved stopper and wash as per Eu.Ph. and fill with EDTA solution. In pencil, identify the sample by writing on the beaker’s external surface, cover the beaker with glass stopper (plate).
Once the autoclave is filled with samples, introduce a data logger and start a sterilizing cycle. At the end of the process, open the autoclave and let the sample cool at a natural rate.
- Glass Tubing: collect 10 ml of extract from each tube in a separate glass beaker, add some drops of Acetic Acid and analyze by AAS.
- Glass cartridges: collect 10 ml from the set of cartridges, add some drops of Acetic
- Acid and analyze by AAS.
An appropriate set of calibration standards have to be put in place, in the same manner as the final sample also considering the addition of acid.
Limits
The results obtained from the experimental routine identified a realistic limit of leached Al concentration, valid for AAS and especially for the ICP technique, which allow to discriminate among different glass tubing manufacturing processes.
Based on experimental data and repeated tests, the inner surface leaching of Al appears to be not as affected by the converting process (cartridges have not a closed glass bottom) and consequently, if applied correctly, the described methodology results effective on the glass tube and on the cartridges in the same way.